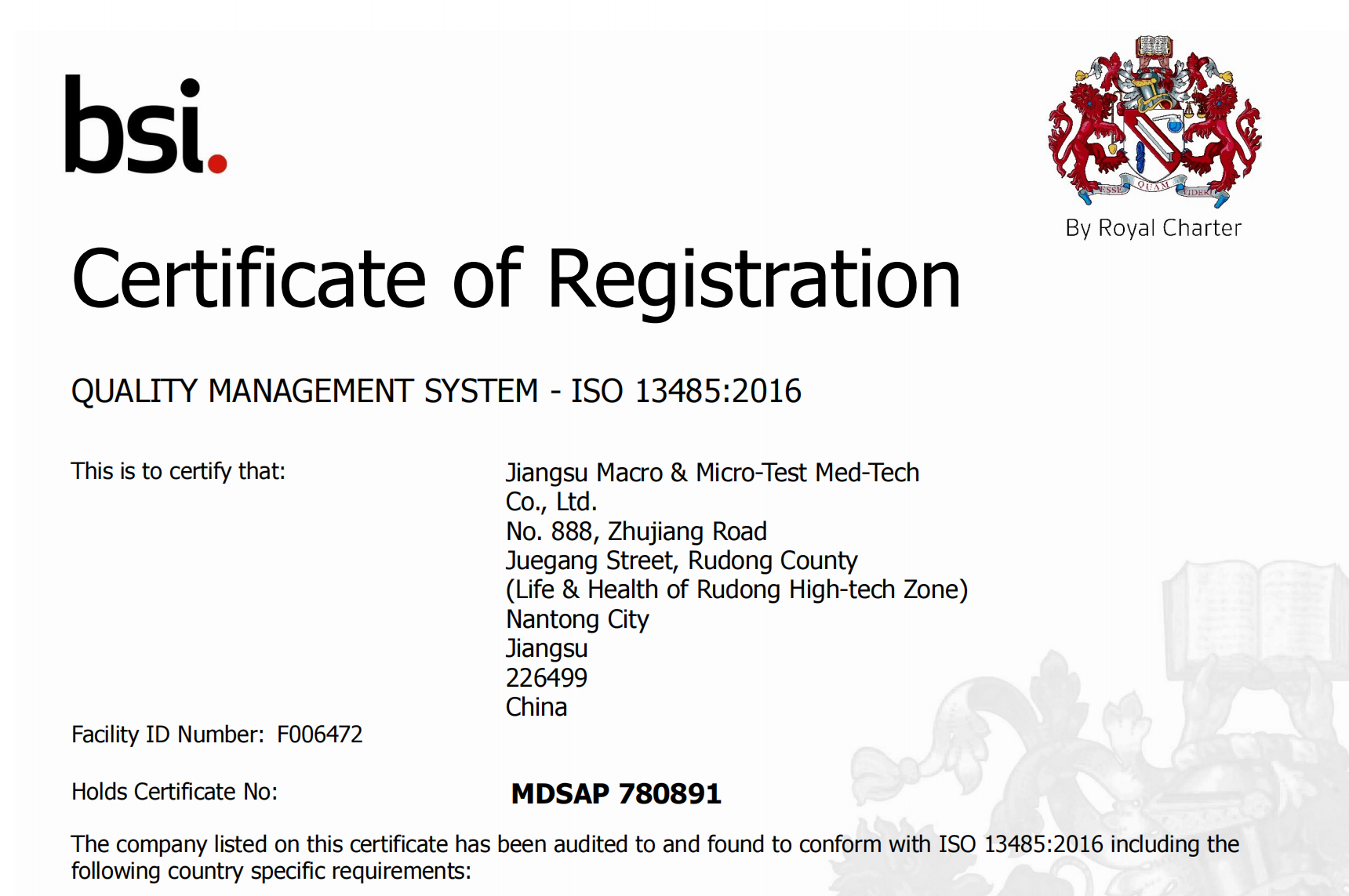
Peb zoo siab tshaj tawm tias peb tau txais daim ntawv pov thawj Medical Device Single Audit Program (#MDSAP). MDSAP yuav txhawb nqa kev pom zoo ua lag luam rau peb cov khoom hauv tsib lub tebchaws, suav nrog Australia, Brazil, Canada, Nyiv Pooj thiab Tebchaws Meskas.
MDSAP tso cai rau kev ua ib qho kev tshuaj xyuas kev cai lij choj ntawm lub chaw tsim khoom siv kho mob lub kaw lus tswj hwm zoo kom ua tau raws li cov kev cai ntawm ntau lub koom haum tswj hwm lossis cov tub ceev xwm uas ua rau muaj kev saib xyuas kev cai lij choj ntawm cov chaw tsim khoom siv kho mob lub kaw lus tswj hwm zoo thaum txo qis kev nyuaj siab rau kev lag luam. Qhov kev pab cuam tam sim no sawv cev rau Australia's Therapeutic Goods Administration, Brazil's Agência Nacional de Vigilância Sanitária, Health Canada, Nyiv Ministry of Health, Labour thiab Welfare thiab Pharmaceutical and Medical Devices Agency, thiab US Food and Drug Administration's Center for Devices and Radiological Health.
Lub sijhawm tshaj tawm: Plaub Hlis-13-2023