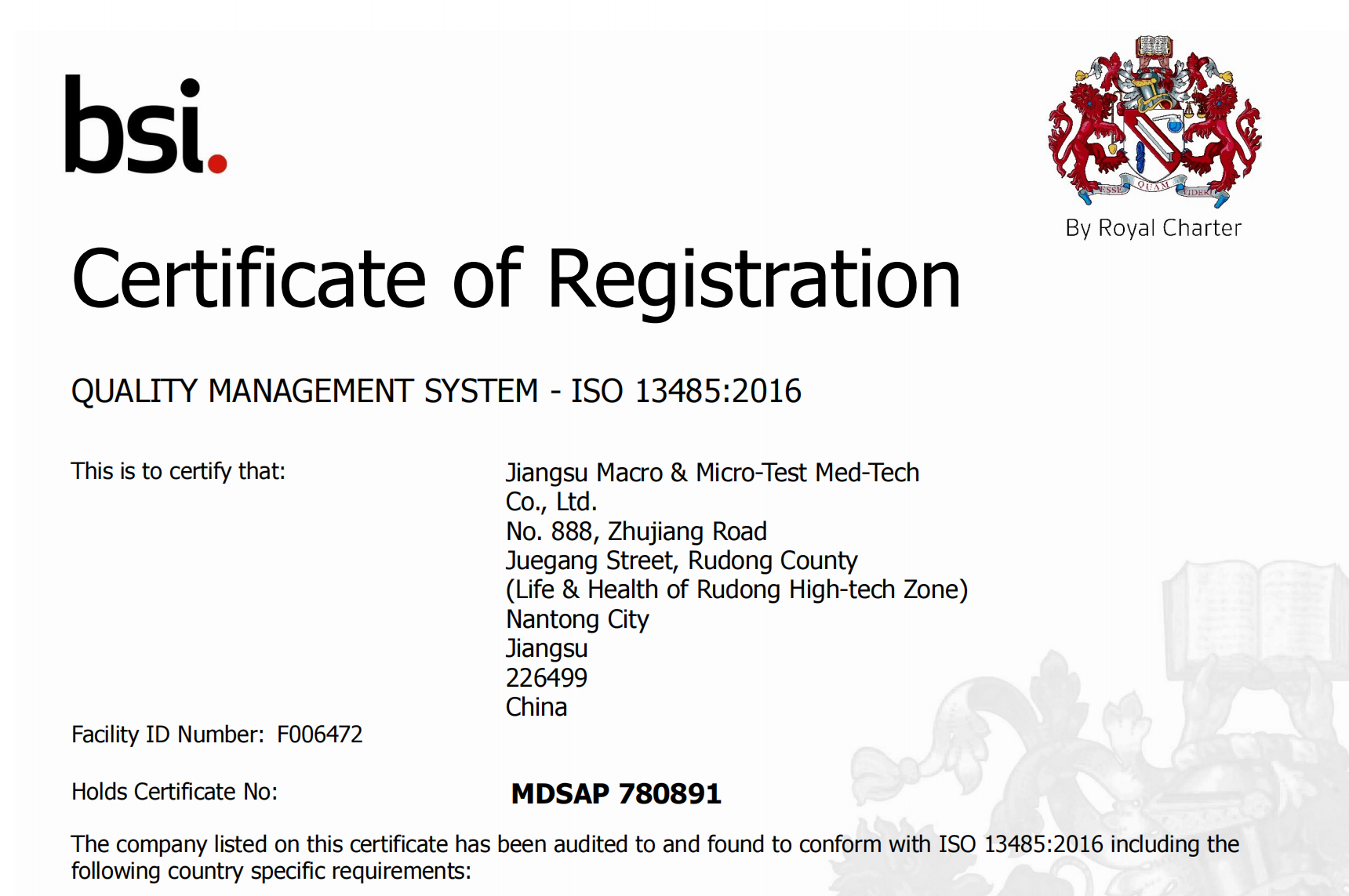
Peb zoo siab tshaj tawm tau txais daim ntawv pov thawj Kev Kho Mob Ib Leeg Kev Tshawb Fawb (#MDSAP).MDSAP yuav txhawb nqa kev pom zoo ua lag luam rau peb cov khoom hauv tsib lub tebchaws, suav nrog Australia, Brazil, Canada, Nyiv thiab Asmeskas.
MDSAP tso cai rau kev coj ua ntawm ib qho kev soj ntsuam ntawm ib lub chaw tsim khoom siv kho mob cov kev tswj xyuas zoo kom ua tau raws li qhov yuav tsum tau muaj ntawm ntau txoj cai tswj hwm lossis cov tub ceev xwm ua kom muaj kev saib xyuas tsim nyog ntawm cov cuab yeej siv kho mob cov cuab yeej tswj xyuas kom zoo thaum txo qis kev tswj hwm lub nra ntawm kev lag luam.Qhov kev pab cuam tam sim no sawv cev rau Australia's Therapeutic Goods Administration, Brazil's Agência Nacional de Vigilância Sanitária, Health Canada, Nyiv Ministry of Health, Labor thiab Welfare thiab Pharmaceutical and Medical Devices Agency, thiab US Food and Drug Administration's Center for Devices and Radiological Health.
Post lub sij hawm: Apr-13-2023